

With the CE Mark, the company can start European-wide sales and marketing of the company’s innovative first catheter, indicated for men experiencing chronic, non-neurogenic urinary retention.
A disruptive new device which could help reduce the billions1 spent by the NHS and the EU healthcare systems on treating Catheter-Associated Urinary Tract Infections (CAUTIs) has received European marketing authorization today. In all, 15%-25% of hospitalized patients and 5% of residents in elderly homes in Europe have a urinary catheter.2
cymactive™ has been designed with the patient in mind to provide a better solution compared to both Foley-type catheters and intermittent catheters. The four primary benefits the company intends to demonstrate are:
cymactive™ uses a Malecot anchor, a four-winged flange, in the bladder with drainage at the base to allow patients to have a complete void while urinating. cymactive™ is approved to remain securely in-situ for up to 30 days. With cymactive™’s innovative UroValve™, a patient or patient’s carer uses an external magnet to activate a valve in the catheter within the urethra allowing the natural and complete emptying of the bladder at the patient’s convenience. Entirely internal, cymactive™ is discreet with only two slender removal threads extending from the penis. This design combines the stability of a Foley catheter with the autonomy and bladder function of an intermittent catheter.
Ingenion Medical’s CEO, Mr. Edward Cappabianca, comments:
“We’re excited that the CE Mark means we’re now able to move into a new phase of introducing cymactive™ across the EU, including the company’s home market of the UK.
Our belief is that cymactive™ will be hugely preferable to men than current options in terms of their quality of life, with the potential for reduction of CAUTIs. With cymactive™, not only do men not have to wear a bag, which can be embarrassing, but our previous work has shown that cymactive™ users have full confidence in the device: they feel they are once more in control of their own urination.
“In the longer term, we aim to demonstrate the full potential of cymactive™ in terms of tackling the significant issue of CAUTIs. By demonstrating a reduction in these infections, we can help urologists to improve care and outcomes, reduce mortality, antibiotic use, and hospital re-admissions, thereby saving the health systems across Europe significant amounts of money currently spent on treating these infections.”
Ingenion Medical’s Commercial Director, Tim Maloney, says: “cymactive™ has been designed to improve safety and quality of life while reducing costs for health and social care providers. Now we have the CE Mark, we look forward to showing details of our product’s full benefits to government healthcare systems. We believe this is a pivotal moment for the company.”
Millions of people worldwide suffer from chronic conditions that impact their ability to lead normal lives because those conditions affect their ability to control their own urination. From men with Benign Prostatic Hyperplasia to people with spinal cord injuries, Parkinson’s Disease, Multiple Sclerosis, and Spina Bifida, the use of both Foley-type and intermittent catheters in the community contribute to a significant percentage of CAUTIs worldwide.3 One market leader estimates that a long-term catheter user, experiences 2.7 CAUTIs per year on average,4 which is both costly and damaging to patients’ long-term health outcomes. Ingenion’s goal is to reduce the incidence of infections, improve quality of life, while also significantly reducing the amount of waste associated with both Foley-type catheters, as well as intermittent catheter waste. The value of the global catheter market was estimated by two leading industry analysts as worth US$5.5 billion (UK Pounds £4.3 billion) in 2023.5
Commenting on cymactive™, Professor Chris Chapple, Emeritus Consultant Urological Surgeon, Sheffield Teaching Hospitals NHS Foundation Trust, Honorary Professor, University of Sheffield, and Visiting Professor, Sheffield Hallam University, says: “There is a significant unmet need for men relating to intervention following urinary retention. Many men would prefer not to have either an indwelling catheter or use intermittent self-catheterisation. Potentially, this device will prove to be a very effective alternative solution.”
Ingenion Medical’s Chairman, Dr. Sergio Rothstein, says: “We are planning to take our core, patented valve technology into multiple use cases, including solutions for women’s incontinence and retention. Therefore cymactive™ is the first of a pipeline of world-class, disruptive urology solutions from Ingenion.”
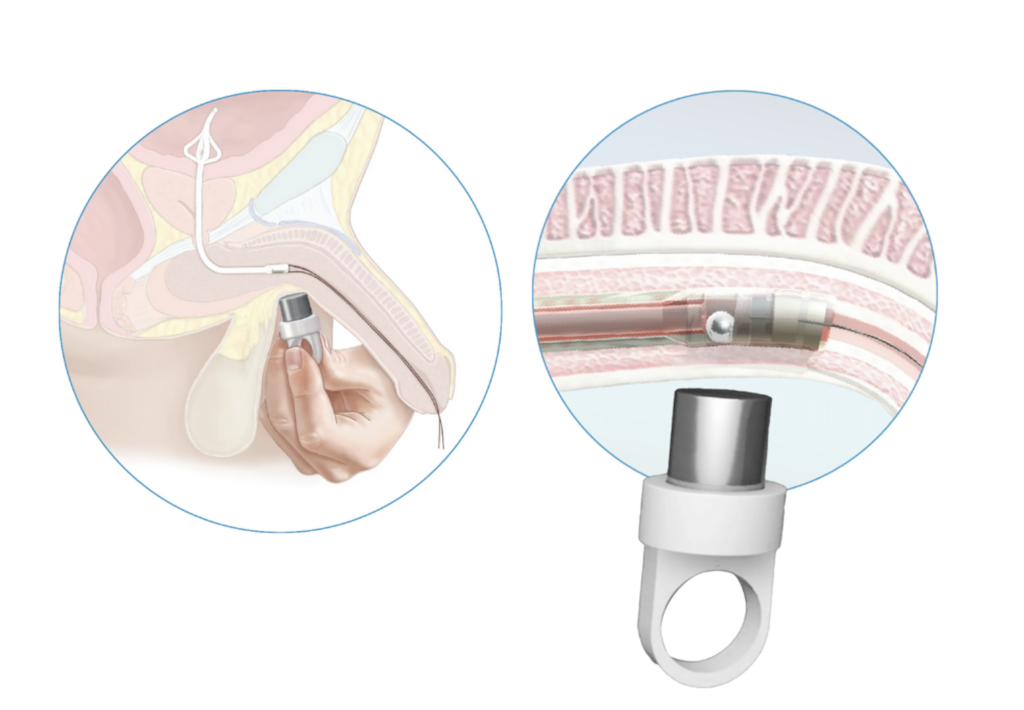
Above and left: cymactive™’s innovative UroValve™, showing how an external magnet activates a valve in the catheter within the urethra allowing the natural and complete emptying of the bladder at the patient’s convenience.
About Ingenion Medical Limited
Privately held, UK-based Ingenion Medical Limited is developing, manufacturing, and commercializing a family of products for chronic urinary retention and incontinence patients. cymactive™, its lead product, is a novel urinary catheter which can remain in-dwelling for up to 30 days, a dramatic quality of life improvement versus legacy solutions that often require multiple catheters in a single day, a common cause of infection and source of medical waste. Visit us at: ingenionmedical.co.uk.
Media contact
Amanda Hayhurst, Gloucester Road Communications Ltd. Tel: +44 772 0205581. E: amanda@amandahayhurst.com
Ingenion contact
Tim Maloney, Commercial Director. E: info@ingenionmedical.co.uk


